Abstract
Background: Most MDS patients with del(5q) develop RBC TD or need treatment for symptomatic anemia early after diagnosis (median time to transfusion/treatment of 20 months, López Cadenas et al abstract 3180 ASH 2016). Lenalidomide (LEN) is a reference treatment in MDS-del(5q) but approved in many countries only when RBC-TD occurs. No randomized trials before Sintra-Rev had explored the role of LEN in the setting of non-TD anemia in del(5q) MDS. Interim analysis of Sintra-Rev (Lopez Cadenas et al abstract 536 ASH 2020) showed that early treatment with LEN prolongs the Transfusion Free survival (p=0.021), with a high percentage of erythroid (72%) and cytogenetic responses (80%) among LEN treated patients.
Material: Sintra-Rev is a phase III multicenter trial in low-risk MDS-del(5q) patients with anemia without TD. Patients were randomized (2:1) in a double-blind design to LEN (5mg/day continuously) vs placebo for 2 years of treatment and 2y of follow-up. The primary endpoint was the time to TD. 2º endpoints included erythroid (HI-E), cytogenetic response (CyR), overall survival (OS), event free survival (EFS), time to AML and mutational analysis (NGS). Here, we report the final results of this clinical trial once all patients have completed their participation in the study and after extending follow-up to explore long-term AML transformation and/or death.
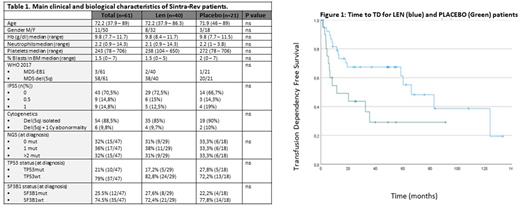
Results: Main clinical characteristics of the 61 patients (ITT population) included are summarized in Table 1: In 32% of patients no somatic mutation was identified (NGS), compared to 36% with one mutation and 32% with ≥ 2 mutations. TP53mut was present at diagnosis in 17.2% and 27.8% of LEN and Placebo patients (p=0.48). The evaluable population included 54 patients (7 patients were excluded due either to screening failure [1 pat] or failure to complete at least 12w of treatment [6 pat] without progression disease). Median time on treatment was 66 weeks (3-121), 95w in the LEN arm and 42w in the placebo arm (p=0.392). Forty-seven percent patients in the LEN arm successfully completed the study compared to 33% in the placebo arm. After a median follow up of 60.6 months (Q1 32.3-Q3 73.9), median OS was 101.2m (95CI 54.8 - 147.7) without statistically significant differences between the two treatment groups (p=0.529). In relation to the primary objective of this trial, Time to TD (ITT-pop) was 66.3mo (95CI 37 - 95.5) for LEN patients and 11.6mo for the placebo arm (HR 0.414 95CI 0.196- 0.875, p=0.021) (Figure 1). EFS was 60.2mo (CI95% 45-74) for LEN compared to 20.2mo (CI95% 0 - 51) for Placebo (p=0.058) in the ITT Population (HR 0.46, CI95% 0.2 - 0.948, p=0.035 in the evaluable population). HI-E was observed in 70% of LEN patients (ITT-pop) compared to 0% in the placebo arm (p<0.001). Median Hemoglobin improvement in responders was 2.7 g/dL. Eighty percent of LEN patients (ITT-pop) achieved a cytogenetic response (CyR) (87.5% complete CyR) compared to 0% of patients receiving placebo (p<0.001). AML evolution was identified in 6/40 (15%) and 5/21 (23.8%) for LEN and Placebo patients respectively (p=0.488). NGS analysis during follow up showed the mean number of mutations per patient to be reduced in the LEN arm at week 12 (0,84 mutations compared to 1,21 previous to treatment; (p<0.01)), while no differences were found in the Placebo group (p=0.59). In addition, no differences were identified regarding clonal evolution for LEN and Placebo patients during study follow-up. Fifty-eight patients developed at least one adverse event (AE) during the trial (no differences between the LEN and placebo arm), related to the drug 86.8% in the LEN and 33.3% in the placebo arm, respectively (p<0.001). Severe AEs were the same as those collected in the interim analysis (abstract 536 ASH 2020).
Conclusions: This final analysis of the Sintra-Rev clinical trial confirms that low dose LEN (5 mg), in anemic non-TD low risk MDS del(5q) patients, prolongs time to TD from the beginning of treatment (66.6 mo vs 11.6mo), improves Hb levels (70% of ER) and induces good quality clonal responses (80% CyR), ie potentially more responses than in historical series of MDS del(5q) treated with LEN at time of TD, with a manageable safety profile, and no increased progression rate or clonal evolution.
Disclosures
Hernández-Rivas:BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Rovi: Membership on an entity's Board of Directors or advisory committees; Takeda: Speakers Bureau; Lilly: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sanz:Abbvie Pharmaceuticals: Other: Advisor or review panel participant; La Hoffman Roche Ltd.: Other: Advisor or review panel participant; Novartis Oncology: Consultancy; Celgene Corporation: Consultancy; Janssen Pharmaceuticals, Inc.: Other: Teaching and Speaking; takeda: Honoraria; Helsinn: Honoraria, Other: Advisor or review panel participant; Takeda Pharmaceuticals Ltd: Other: Advisor or review panel participant. Giagounidis:BMS: Honoraria. Goetze:Abbvie: Honoraria; Servier: Honoraria; BMS: Honoraria. Hernández Rivas:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Fenaux:Jazz: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria. Diez-Campelo:Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; BluePrint: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Use of LENALIDOMIDE in non transfusion dependency low risk del(5q) MDS patients. Its use is approved in this entity but in Transfusion dependency setting.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal